The Kenya Medical Research Institute (KEMRI) on Tuesday announced the commercialisation of its COVID-19 PCR and malaria Rapid test Kits.
Kenya Medical Research Institute @KEMRI_Kenya has today put Kenya on the global research map following the launch of two major innovations namely: Covid-19 PCR testing kit and Malaria detection testing kit. pic.twitter.com/ByYfB6tONM
— Ministry of Health (@MOH_Kenya) February 15, 2022
“These two kits will go a long way in improving Malaria diagnostics and COVID-19 disease detection, which remain our foremost health challenges not just in Kenya but globally,” Chief Administrative Secretary Ministry of Health Dr Rashid Aman said during the commissioning at the 12th KEMRI Annual Scientific & Health Conference at Safari Park Nairobi.
The two products have been approved for use by the Pharmacy and Poisons Board and will go a long way in reducing the cost of treatment in the country.
Dr Aman urged the Kemri management to hasten the marketing of the kits by having them available in all public and private health facilities so as to lower the cost of the service they will provide.
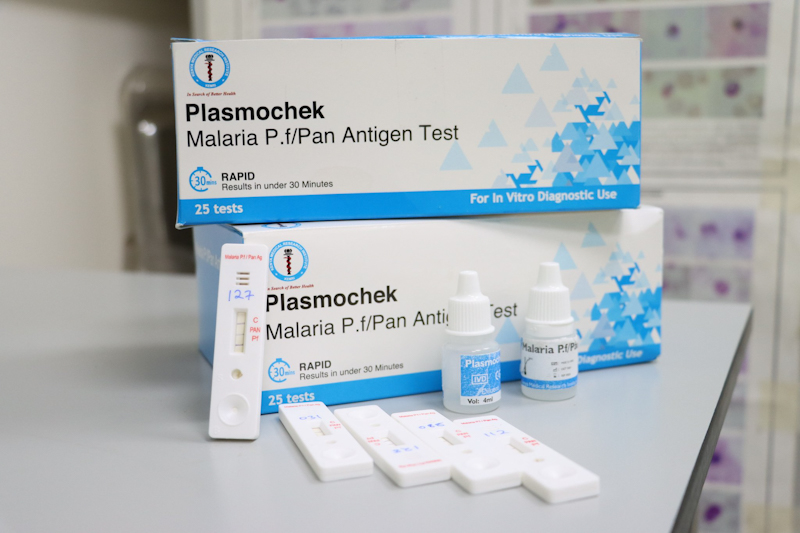
The Malaria Rapid Test Kit (PlasmoCheK) will be instrumental in enabling faster diagnostics & surveillance of Malaria.
The Malaria Rapid Test Kit (PlasmoCheK) will be instrumental in enabling faster diagnostics & surveillance of #Malaria
The Kit is
✅locally produced
✅More affordable & more accessible.
✅100% sensitive & specific
✅Can be performed by untrained or minimally skilled personnel pic.twitter.com/GUtpIQketi— Kenya Medical Research Institute (@KEMRI_Kenya) February 15, 2022
On the other hand, the KEMCov-19 PCR test kit is intended for qualitative pathogen detection and characterization of severe acute respiratory syndrome coronavirus 2″ (SARS-CoV-2) Ribonucleic acid (RNA).
The COVID-19 test will cost Ksh 1,500 as compared to the usual Ksh2,000 in Kenya and at least Ksh 5,000 in public and private facilities respectively.
Ag. Director-General KEMRI, Prof. Sam Kariuki said the two products showcase the capabilities and innovativeness of their scientists in the country.
KEMRI says the two products will save the government KSh58 million for the malaria kits and KSh405 million for the Covid-19 PCR tests annually.
We are on Telegram. Click here to join our channel and stay updated with the latest East African business news and updates.




1 Comment
Pingback: Kenya Among Six African Countries to Receive mRNA Vaccine Technology